Phosphoenolpyruvate/phosphate translocator – Part 2
The role of PEP in plastids
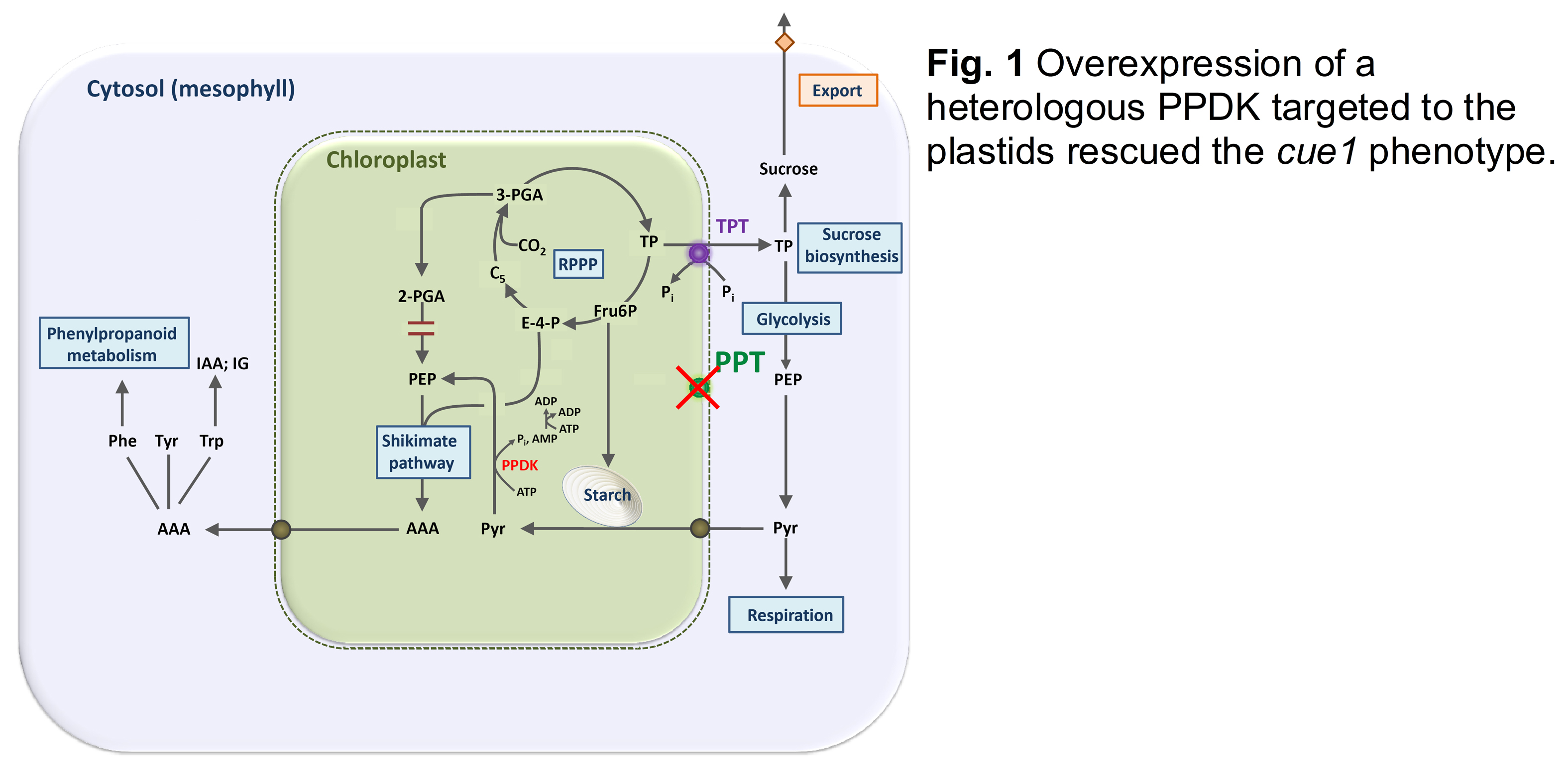
PEP has a central role in plant metabolism (Flügge et al., 2011). In plastids PEP, together with erythrose-4-P (E-4-P), serves as a substrate for the shikimate pathway of aromatic amino acid biosynthesis. In the cue1 mutant (see Part 1) the loss of PPT1 function could be rescued by overexpression of a heterologous pyruvate:phosphate dikinase (PPDK) targeted to the plastids (Voll et al., 2003). PPDK can produce PEP from pyruvate in the stroma and thereby substitutes PEP import in the absence of PPT1 (Fig. 1).
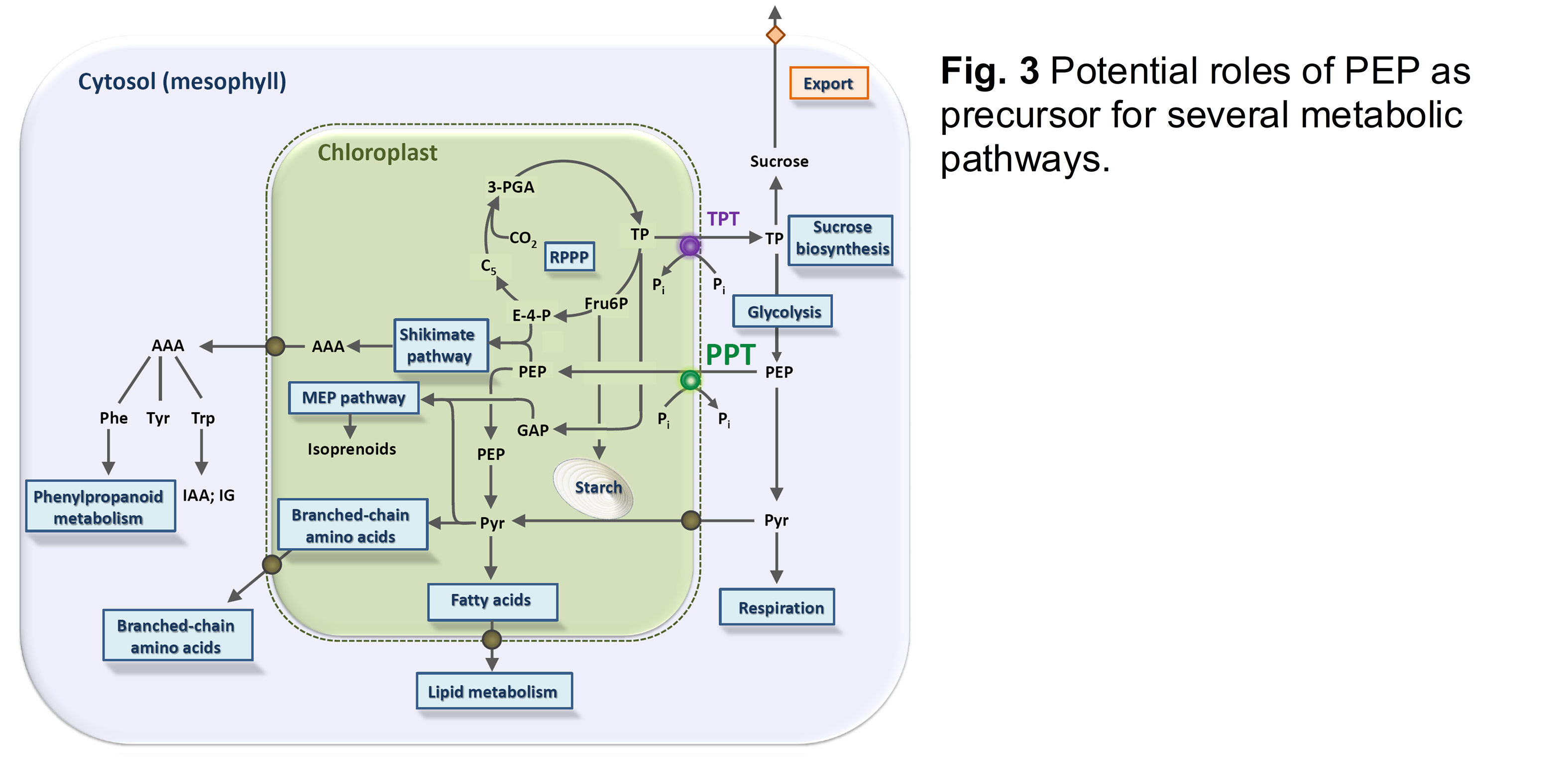
Most plastids (including chloroplasts) lack a complete glycolysis and thus cannot produce PEP from 2-phosphoglycerate catalysed by enolase. In contrast, plastids in reproductive or meristematic tissues as well as in certain root cells contain a plastidial enolase (ENO1; Prabhakar et al., 2009). However, a loss of function of ENO1 in the respective single mutant alleles did not result in a severe phenotype. Only if ENO1 was knocked out in combination with PPT1 the homozygous double mutants were lethal (Prabhakar et al., 2010), underlining the central role of plastidial PEP for survival of the plants. Moreover even heterozygous eno1 mutants in the homozygous cue1 background showed a more severe phenotype compared to cue1 (Fig. 2). From these data it is likely that PEP not only serves as precursor of the shikimate pathway, but, after conversion to pyruvate, also for de novo fatty acid biosynthesis, the methylerythritol phosphate (MEP) pathway of isoprenoid biosynthesis or the synthesis of the branched chain amino acids Leu and Val (Fig. 3).
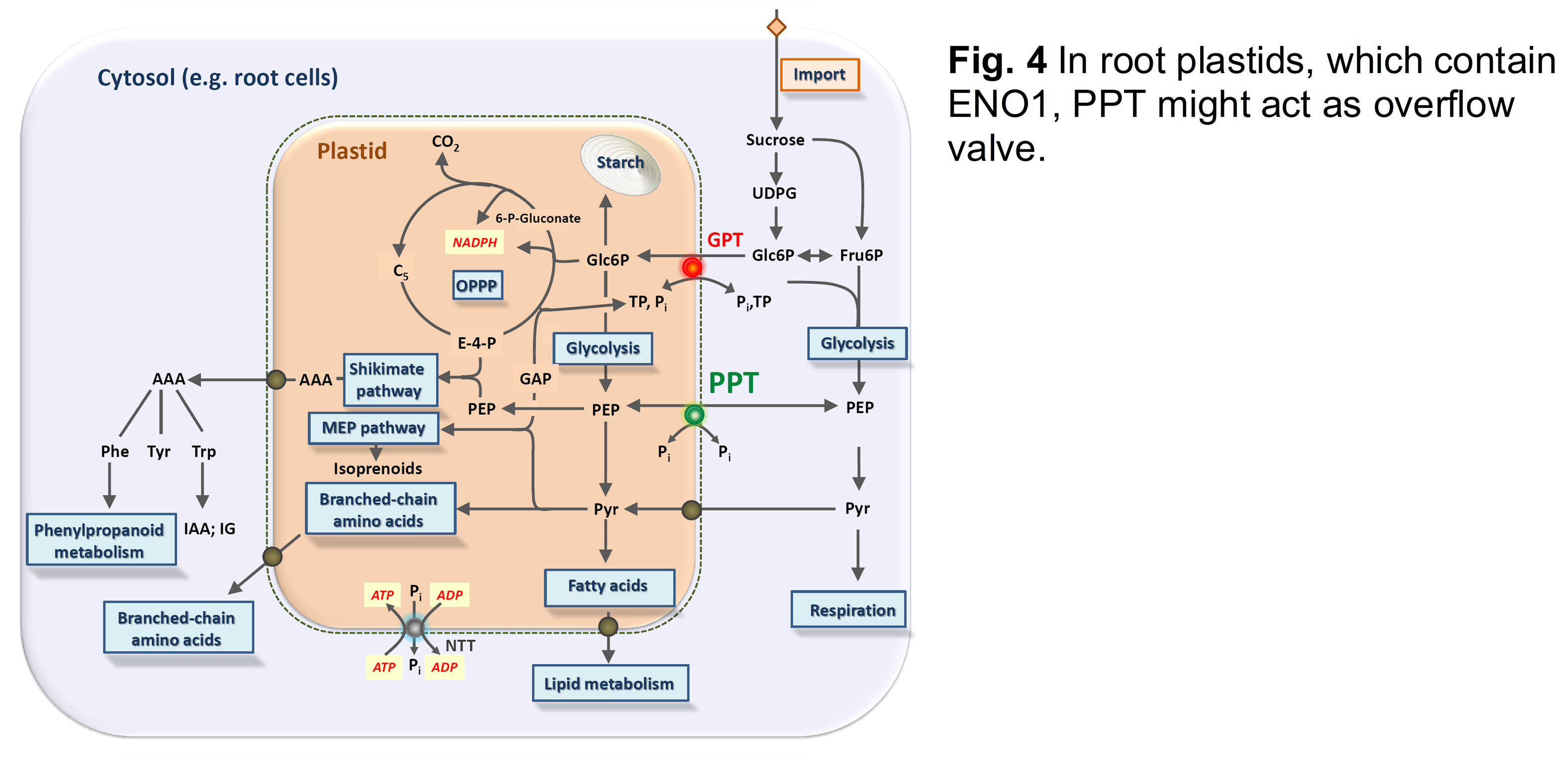
The shoot and root phenotypes of the cue1 mutant were further dissected by grafting experiments as well as feeding of the cytokinin trans-zeatin or the phenylpropanoid derivative dehydrodiconiferyl alcohol glucoside (DCG; Staehr et al., 2014). In plastids of root cells, which can produce PEP by glycolysis (Prabhakar et al., 2009), the PPT might operate as overflow valve between cytosol and stroma (Fig. 4)
(back to Rainer E. Häusler)